Alzheimer’s Disease (AD) is a prime topic for research due to its vast impact on memory and cognitive decline. In recent years, there has been a surge of information regarding AD research, including reports of fraud from highly cited studies and a new potential treatment. The following article will break down these aspects and offer insight into the future of AD research.
Allegations of Fraud
A defining feature of AD research is the presence of large protein clumps in the brain. These proteins, known as amyloid-beta proteins, are commonly produced by cells and tissues throughout the body. Normally, amyloid-beta protein clumps are broken down and removed from the brain constantly. However, this clearance process slows down among AD patients as these proteins become less soluble, resulting in larger amyloid-beta plaques in the brain.
A high-impact Nature paper from 2006 reported that a specific aggregate of amyloid-beta proteins (amyloid-beta*56) played a key role in cognitive decline. Therefore, a lot of research built off the assumption that amyloid-beta*56 had a specific and crucial role in AD. However, in 2022, reports of fraud in the original paper surfaced. A few researchers accused the original authors of data manipulation, causing concerns in general science credibility and the utility of the studies that built off of the original research.
These allegations brought great concern to AD research, but all was not lost. Although credibility regarding the specific role of amyloid-beta*56 in AD is questionable, the general amyloid-beta hypothesis on AD is still well-supported. The consensus that amyloid-beta plaques are a defining feature of AD was not established by a single study. Rather, thousands of publications from countless researchers helped cement this defining feature of AD.
A New Treatment for AD
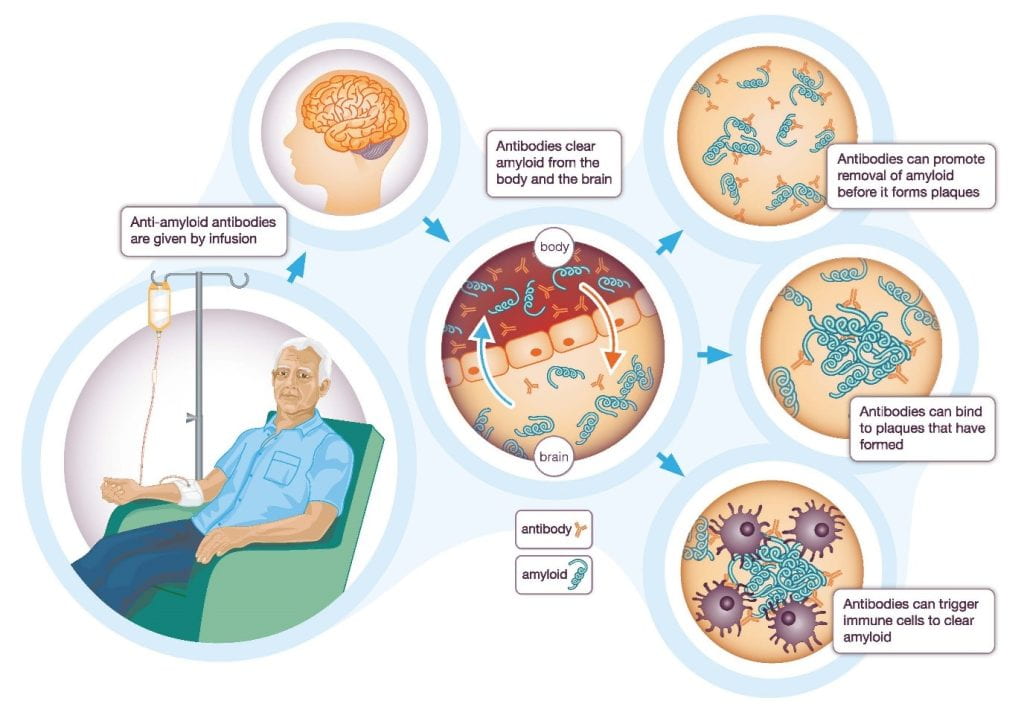
In 2023, the general public was informed of a new drug that reduces cognitive decline in AD. Lecanemab is an antibody that binds to amyloid-beta plaques, triggering immune responses that remove these protein plaques. This drug is thus believed to slow down the progression of early AD symptoms. Although lecanemab is not a cure for AD, its potential is great progress for AD research.
After multiple clinical trials from the last decade, lecanemab was FDA-approved and marketed in the United States in 2023. Based on its clinical results, lecanemab removed amyloid-beta plaques and alleviated cognitive decline among early AD patients. However, further studies are still needed to indicate whether lecanemab could be used to treat patients in the late stages of AD.
Future of AD Research
As shown by the allegations regarding amyloid-beta*56, scientific research requires credibility to initiate progress. Although this fraudulent act may have backed up and troubled years of AD research, work such as lecanemab continues to surface in the present day.
AD research is complex and is built on decades of work. However, as long as credible and reproducible AD research is produced, the scientific community will continue to draw closer to a cure for AD.
Sources
- https://memory.georgetown.edu/news/allegations-of-fraud-in-alzheimers-disease-research-death-of-the-amyloid-hypothesis%EF%BF%BC/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10196238/
Images
You must be logged in to post a comment.