On Friday, April 5, the Nobel Prize in Chemistry winner Moungi Bawendi lectured at Amherst College. The lecture was held in Lipton lecture hall and was titled “Quantum magic and quantum dots: a synthesis unlocks a nano-world of opportunities.”

Bawendi opened his talk by explaining how electrons have different properties at the quantum level.
“So when we think about electrons in a circuit, we think of a little particle of charge that’s moving around. If you take an electron and try to stuff it in a small box, […] that electron no longer behaves like a charge that moves around. Now, it behaves more like the electron in a hydrogen atom; it acquires wave-like properties,” explained Bawendi.
Traditionally, electrons behave like tiny charged particles. However, once electrons are confined to a really tiny space (e.g., a box on the nanoscale), they start acquiring wave-like properties, known as quantum confinement. This box is a semiconductor particle, also known as a quantum dot.
The box’s size highly affects the electron’s energy: smaller waves have higher energies, and larger waves have lower energies. How do quantum dots release energy and emit light? One can experience this by shining light on quantum dots to excite electrons from a lower to a higher energy state. After a short period, these excited electrons return to their original energy levels, emitting light. Higher energy electrons produce higher energy photons (emits blue light), and lower energy electrons produce lower energy photons (emits red light). Therefore, one can tune the size of quantum dots to produce every color in the rainbow.

Bawendi received the 2023 Nobel Prize in Chemistry with Louis E. Brus and Alexei Ekimov for discovering and synthesizing quantum dots. In the 1980s, Brus and Ekimov discovered the quantum size effect in semiconductors. Bawendi’s work was largely devoted to the synthesis of these quantum dots. In 1989, Bawendi worked on a project involving semiconductor particle syntheses and found a sample that emitted light in a previously unseen way. This sample was created from soap, oil, and various ions, and its size could be controlled by its reaction conditions.
However, when he arrived at MIT, Bawendi and his colleagues struggled to reproduce these nanoparticles. The synthesis protocol was modified by raising the reaction temperature, using organometallics, keeping the reaction air-free, and much more. These modifications were known as the “hot injection method.” This protocol was optimized into a simple reaction, providing a reproducible synthesis for quantum dots.
Bawendi’s first samples had a quantum yield (the ratio of the number of photons emitted to the number of photons absorbed – a quantitative measure of light emission efficiency) of 15-20%. Nowadays, through the scientific community’s work, the quantum yield is up to 100%.
Quantum dots have many applications, especially among technology companies, for commercial use. The first application was in biomedical imaging. Since quantum dots are highly fluorescent objects the size of a protein, they were used to label different cell parts. Unlike fluorescent dyes, quantum dots are less susceptible to photobleaching (when a molecule permanently loses its fluorescent ability due to chemical damage).
The next application was in lighting and displays. Early LED televisions had three color LEDs (blue, red, and green), but purchasing three different LEDs was expensive for large-scale production. In response, companies placed red and green emitting quantum dots on top of blue LEDs, eliminating the need to purchase red and green LEDs. Companies like Sony and Samsung produced quantum dot televisions that were highly profitable.
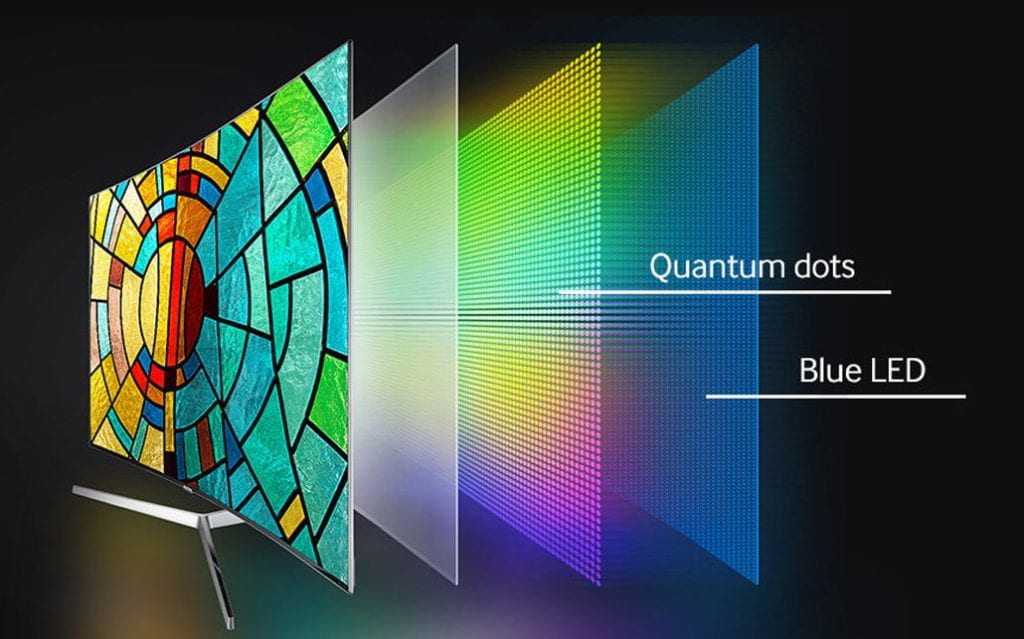
Quantum dots continue to have a wide range of applications. Some common applications today include their use in MRI imaging, new light bulbs, and solar cells. Thanks to the hard work, dedication, and curiosity of Brus, Ekimov, and Bawendi, quantum dot applications are still actively studied and researched today.
Bawendi stated, “We came from this observation and being really curious about materials. With the quantum effect and the magic that quantum mechanics can venture, […] I had no idea there would be applications that would come out of it. And today, we’re everywhere.”
Images:
https://www.nersc.gov/news-publications/nersc-news/science-news/2015/quantum-dot-blinking/
https://www.lifewire.com/quantum-dots-enhance-lcd-tv-performance-1847342

You must be logged in to post a comment.